Manufacturing Execution in the Pharmaceutical Industry
The pharmaceutical industry plays a critical role in providing the medicines and treatments that keep people healthy and save lives. However, the complex and highly regulated nature of pharmaceutical manufacturing requires precise and efficient processes to ensure product quality, safety, and compliance with regulatory standards. This is where manufacturing execution systems (MES) come into play.
MES is a software platform that manages and monitors the manufacturing process in real-time, from raw material tracking to final product release. MES helps ensure that all the manufacturing steps are completed correctly and on time, while also providing accurate and reliable data for regulatory compliance and continuous improvement.
Benefits of MES in the Pharmaceutical Industry
The pharmaceutical industry plays a critical role in providing the medicines and treatments that keep people healthy and save lives. However, the complex and highly regulated nature of pharmaceutical manufacturing requires precise and efficient processes to ensure product quality, safety, and compliance with regulatory standards. This is where manufacturing execution systems (MES) come into play.
MES is a software platform that manages and monitors the manufacturing process in real-time, from raw material tracking to final product release. MES helps ensure that all the manufacturing steps are completed correctly and on time, while also providing accurate and reliable data for regulatory compliance and continuous improvement.
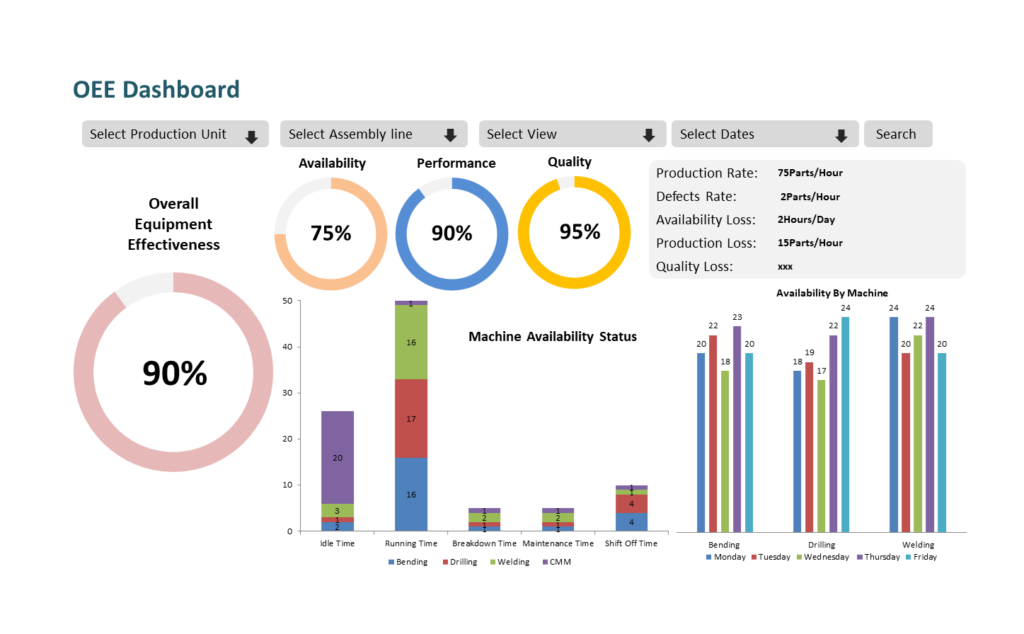
- Real-time monitoring and control
MES provides real-time visibility into the manufacturing process, enabling operators to monitor and control critical parameters such as temperature, humidity, and pressure. This helps ensure that the process is running within specified parameters and allows operators to take corrective actions immediately if there are any deviations. - Improved traceability
MES tracks the movement and usage of raw materials, equipment, and personnel throughout the manufacturing process. This provides full traceability of the product, from raw materials to finished goods, ensuring compliance with regulations and helping to quickly identify the root cause of any quality issues. - Enhanced quality control
MES can automatically enforce quality control checks at each step of the manufacturing process, such as in-process testing and inspection. This helps ensure that products are manufactured according to the specifications and standards, reducing the risk of defects and recalls. - Increased efficiency and productivity
MES streamlines manufacturing operations by automating routine tasks and reducing manual data entry. This allows operators to focus on value-added tasks and makes the manufacturing process more efficient, reducing lead times and increasing production throughput. - Better regulatory compliance
MES provides accurate and reliable data for regulatory reporting and audits. It can track all the required data, such as batch records, production history, and environmental conditions, making it easier to demonstrate compliance with regulatory standards.
Conclusion
The pharmaceutical industry is highly regulated, and compliance with regulatory requirements is essential. Manufacturing execution systems (MES) have become an essential tool for pharmaceutical companies to manage the complexity of the manufacturing process and ensure compliance with regulatory requirements. MES systems provide a centralized platform that can help to automate and manage the entire manufacturing process, from raw material procurement to finished product delivery. By providing real-time monitoring, automated data collection, and audit trails, MES systems can help to improve efficiency, reduce the risk of errors, and ensure compliance with regulatory requirements.
